Choosing the right solution for the management and tracking of your surgical assets is just the first step in embracing automation. Whether you’re considering asset management automation, already have a system in place, or are awaiting implementation of your new solution, choosing your scan points can make or break the positive impact the solution can have on your department and facility. Following these best practices for finalizing your instrument tracking scan points can help ensure that your facility is set up to efficiently manage its surgical assets. This week we’ll be outlining minimum instrument tracking scan points in decontamination, set assembly, sterilization, storage, case cart assembly, and the operating room (OR) and next week, we’ll be exploring additional scan points that create our complete scan points best practice list.
Decontamination
Scan Point: Scanning case carts and trays to the decontamination receipt. Scanning at this location takes ownership of carts and trays coming into the SPD department. It accounts for all items returning to or arriving at the SPD and ensures that nothing got lost in the transport of OR to SPD after use on a case. Scanning at this point also allows for accurate data on reprocessing time from the point of decontamination receipt to when sets are ready for use again.
Set Assembly
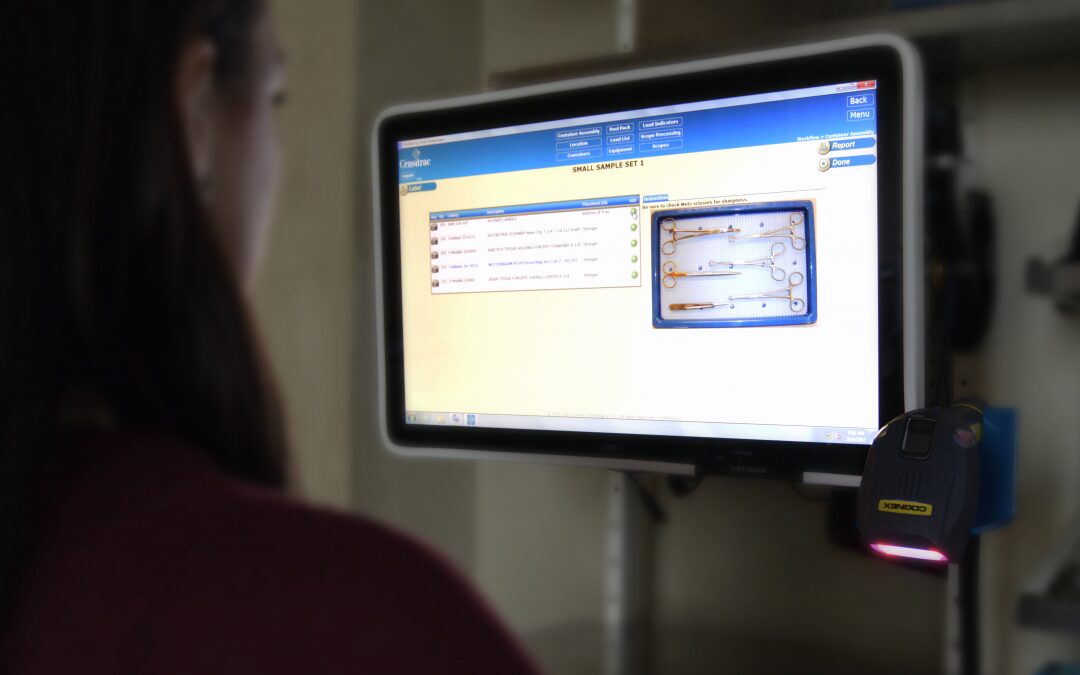
Scan Point: Scanning instruments and trays for container assembly Scanning your instruments and trays for container assembly is an absolute must. In fact, it’s one of the main motivations for facilities when considering adopting surgical asset management automation. With this scan point in place, SPD teams get their count sheets to build trays, access imaging for further detail on assembly, and get special messages and instructions on how to build the tray, which then verifies that the correct instruments go into the correct tray. Utilizing this scan point also enable notifications to alert the department when instruments and trays need maintenance.
Sterilization
Scan Point: Scanning trays to the sterilizer Each sterilizer gets its own barcode that identifies which type of sterilizer it is and what its cycle parameters are. This is critical because in utilizing this scan point, you can be sure that the correct items are going into the correct sterilizer since the system generates an alert, preventing technicians from proceeding whenever a they scan the wrong item or the wrong sterilizer. This scan point also adds additional layers of protection by guaranteeing that all instruments have been properly sterilized. Scan Point: Load results recording Utilizing this step in your asset management solution can help you keep and store all of your sterilization documentation electronically, enabling you to move away from paper record keeping—a huge advantage since facilities are required to keep all records for at least seven years. For example, our automated asset management solution, CensiTrac, builds loads as you scan all of your trays into the sterilizer and then stores all your sterilizer records/tapes from those sterilizer loads. Plus, with the sterilizer interface in place, all of your load results get automatically sent to and stored in CensiTrac. And even if you don’t have a sterilizer interface, you can utilize a document scanner to scan your records right into the solution, eliminating the need for maintaining paper records. Scan Point: Indicator results recording Biological indicators (BI) help facilities ensure that their sterilizers are working correctly. While regulation requires facilities to run a BI in the first load of every day and whenever a load is run that includes implants, many facilities are running BIs for every load. Running a BI in every load ensures that, in the event of a recall, the facility has full documentation of exactly where the contamination originated and limits the number of instruments and trays that may need to be recalled. To aid with the accurate documentation of BI results, our system has parameters in place that verify that a control has been run for every lot number for every machine for every day. Our system also verifies that the BI is put into the correct incubator and well, and then sends a message to the manager when there is a positive test result. It also stores all of those results electronically, once again eliminating the need for department members to record these results via paper records.
Storage
Scan Point: Scanning trays, peel packs, and other items to the general storage area/room. Following sterilization, scanning to a storage location is important for assets that aren’t being used right away. Doing so accounts for where every instrument and tray is following sterilization.
Case Cart Assembly
Scan Point: Scanning trays, peel packs, and other items to a case cart. Each case cart has its own barcode, so we recommend scanning the trays onto a case cart, because it provides visibility to the exact location of any given tray after it has been assembled to a cart.
Operating Room (OR)
Scan Point: Scanning case carts to the OR Core. Scanning your case carts and trays into this location accounts for assets sitting in a holding area before the case gets started. This gives both the OR and the CSSD visibility to the location of that case cart and its assets while it’s waiting to be utilized, avoiding any potential delays that can come from not knowing the exact location of any given case cart within the OR.
Your Instrument Tracking Scan Points Matter
When planning for instrument tracking scan points in your facility, it’s important to remember that every department, team, and facility is different. We recognize that what works for one facility might not work for another. That’s why the first step of implementing our automated asset management solution is to walk through the facility with the SPD and OR team to see their daily workflow, learn how their departments work, and assess what needs they have. Then we provide customized recommendations built from these minimum requirements to establish scan points that will support rather than inhibit facility workflow and efficiency. To get the complete list of best practice instrument tracking scan points, check out part 2 of this series.